In pharmaceutical manufacturing, delivering a safe, effective, and consistent product is not optional. Process validation is a critical pillar supporting this requirement. But what is process validation, and why is it important in the pharmaceutical industry?
Whether you’re a student, a new Quality Assurance (QA) or Quality Control (QC) professional, or preparing for regulatory inspections, understanding the stages of process validation and their significance in pharmaceutical GMP (Good Manufacturing Practices) is essential.
Also read: Ultimate Guide to Cleaning Validation in Pharmaceutical Industry
What is Process Validation in Pharma?
Process validation in the pharmaceutical industry is defined as:
“The documented evidence that a process consistently produces a product meeting its predetermined specifications and quality attributes.”
For example, if you’re manufacturing 500 mg paracetamol tablets, process validation ensures that each batch reliably contains 500 mg ± permissible limits, is uniformly mixed, and meets the required dissolution rate.
This concept is tightly connected to GMP guidelines, where the emphasis is on building a quality product and ensuring repeatability.
Why is Process Validation Important in Pharma?
- Ensures product quality, efficacy, and patient safety
- Required by regulatory authorities like the US FDA, EMA, and CDSCO
- Minimizes batch failures and reworks
- Enhances production efficiency
- Reduces long-term manufacturing risks
It’s a vital piece of pharma GMP, ensuring that quality is built into every step of production, not just tested at the end.
Types of Process Validation
- Prospective Validation
Conducted before commercial distribution. This is the most common type and is mandatory for new processes. - Concurrent Validation
Performed during routine production of products intended for sale — useful when a product is urgently needed. - Retrospective Validation
Based on historical batch records and past data. Now largely obsolete under modern GMPs. - Revalidation
Required when there are significant changes to a process, formulation, equipment, or facility, and also performed periodically as per site SOPs.
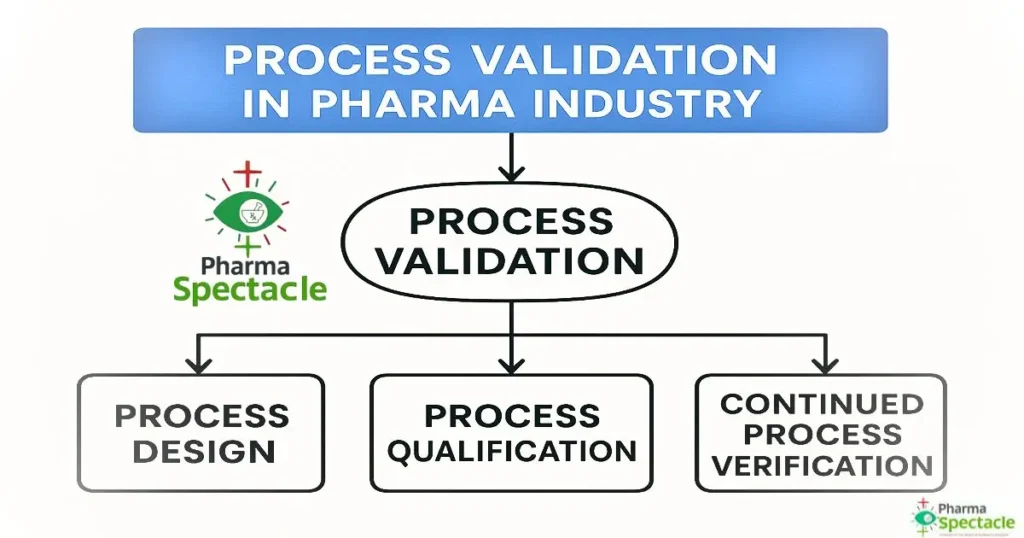
The 3 Stages of Process Validation (As Per FDA Guidance)
The modern lifecycle approach to validation includes three distinct stages, as described in the US FDA’s guidance, and it is adopted globally.
Stage 1: Process Design
This is the development stage where the manufacturing process is designed based on R&D findings, laboratory data & pilot-scale data.
Activities Include:
- Designing equipment and formulations
- Defining critical quality attributes (CQAs) and critical process parameters (CPPs)
- Conducting lab and pilot-scale studies
Example: Optimizing tablet granulation parameters to ensure content uniformity.
Stage 2: Process Qualification
This is where the designed process is tested & evaluated on a commercial scale to prove it consistently works.
It has two subparts:
- Facility and Equipment Qualification: DQ, IQ, OQ, PQ for chosen equipment train if it’s new. If it’s already qualified, then the supporting documents should be attached to the validation protocol.
- Performance Qualification (PQ) — running validation batches (usually 3 consecutive successful ones)
Activities Include:
- Executing validation protocols.
- Challenging CPPs to establish operational limits.
- Sampling and testing of all critical parameters.
- Generating documented evidence.
Example: Running three 100000 tablet batches on a new or old tablet press and showing consistent results for weight, hardness, disintegration, assay, and dissolution.
Stage 3: Continued Process Verification
This stage ensures that the validated manufacturing process remains in a state of control during routine commercial production.
It is essential for detecting any process drift, unexpected trends, or variability over time, which could impact product quality.
It has two subparts:
- Initial Process Verification (IPV)
conducted during the early phase of commercial production, following the successful execution of validation batches. Typically, it involves the evaluation of at least seven consecutive commercial batches in addition to the three validation batches, bringing the total to ten. Data related to Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs) from these batches are compiled and analyzed for trending. The primary objective of IPV is to confirm that the process consistently performs as intended under routine commercial manufacturing conditions.
- Ongoing Process Verification (OPV)
A long-term, continuous activity aimed at ensuring sustained process performance and product quality. It involves routine monitoring, data trending, and the application of Statistical Process Control (SPC) to maintain process capability and regulatory compliance. As part of OPV, data from a minimum of 11 commercial batches are evaluated, in addition to the previously executed 3 validation batches and 7 Initial Process Verification (IPV) batches. In total, 21 batches—comprising 3 validation, 7 IPV, and 11 OPV batches—are compiled for comprehensive data trending and analysis.
Activities Include:
- Real-time data monitoring and control charting of CPPs & CQAs.
- Statistical process control (SPC) and trending critical quality attributes (CQAs) and critical process parameters (CPPs)
- Investigation of any deviations or out-of-trend results
- Annual product quality reviews (APQR)
Example: Regularly analyzing tablet hardness, assay, dissolution, and weight variation data from each production batch to ensure no quality shifts occur over time.
Real-World Example of Process Validation
Let’s consider the manufacturing of an oral tablet like Metformin 500 mg.
| Step | Validation Focus |
| Dispensing | Correct weight and identity of API & excipients. |
| Mixing/Blending | Uniform API distribution – BU assay (assessed by RSD). |
| Granulation | Consistent moisture and granule size. |
| Compression | Tablet weight, hardness, thickness, assay and dissolution. |
| Coating (if any) | Uniform coating thickness and appearance. |
| Packaging | Correct count per bottle/strip, proper labeling. |
Each step must be validated to ensure inter-batch and intra-batch consistency.
Regulatory Guidelines on Process Validation (2025 Update)
Here are the most relevant guidelines recognized globally
| Agency | Guideline Name |
| US FDA | Guidance for Industry Process Validation: General Principles and Practices |
| EMA | Guideline on Process Validation of finished products |
| WHO | WHO Technical Report Series, No. 1019, 2019 (Annex 3) |
| PIC/S | PI 006-3: Recommendations on Validation Master Plan |
| ICH | Q8 (Design), Q9 (Risk), Q10 (Quality System) |
| CDSCO (India) | Schedule M + Draft guidance under review |
Documents Required for Process Validation
- Validation Master Plan (VMP)
- Process Validation Protocol
- Equipment Qualification Reports (DQ/IQ/OQ/PQ)
- Raw Data Sheets and Logbooks
- Analytical Method Validation Reports
- Final Validation Report with Summary & Conclusion
- Trending charts for CPV
Tip: Ensure compliance with ALCOA+ principles to safeguard data integrity.
Common Challenges in Process Validation
- Deviations during validation runs.
- Incomplete documentation.
- Non-validated analytical methods.
- Poorly defined critical parameters.
- Lack of real-time monitoring.
Tip for QA/QC Professionals: Always ensure change control and risk assessments are completed before validation begins.
Frequently Asked Questions on Process Validation
1. How many batches are required for process validation?
Typically, three consecutive batches are used. However, the number can vary based on process complexity and risk.
2. What happens if one validation batch fails?
Root cause analysis is done. Often, the failed batch is discarded, and the process is revalidated.
3. Is process validation mandatory for exports?
Yes. Regulatory agencies like the US FDA, EMA, WHO and others require validated processes for all pharmaceutical products.
4. What is the difference between process validation and cleaning validation?
Process validation ensures the process produces consistent products. Cleaning validation ensures that equipment is free from contamination between batches or products.
5. Can process validation be done using a bracketing approach?
Yes, for similar products or strengths, a bracketing or matrix approach may be accepted. This reduces the number of validation batches by selecting extremes (e.g., lowest and highest strengths) under the assumption that the middle strengths will behave similarly.
Conclusion
So, process validation is much more than a regulatory requirement — it’s a scientific, documented assurance that your process delivers quality, consistency, and safety.
With increasing scrutiny from global regulators in 2025 and beyond, validation practices must align with lifecycle concepts, real-time monitoring, and robust data integrity.
Whether you’re a student or a QA/QC executive, mastering the stages of process validation and its implementation is vital for a successful pharmaceutical career.
Found this post helpful?
- Share it with your friends & colleagues.
- Subscribe to PharmaSpectacle to stay ahead in the pharmaceutical world with valuable pharma knowledge and professional growth tips.
- Join our official channels for the latest government job alerts.
- WhatsApp Channel: Click Here
- Telegram Channel: Click Here
- Got a question? Drop it in the comments below — we reply to every single one!