
DMER Pharmacist Answer Key, Cut-Off and Result Analysis For 2025
Explore category-wise cut-off results predictions for the DMER Pharmacist exam from 2025.

Explore category-wise cut-off results predictions for the DMER Pharmacist exam from 2025.

DMER Maharashtra State Pharmacist Recruitment Exam 2025 dates announced. Check exam schedule, key instructions, and tips for government pharmacist positions.

CSIR-NCL, Pune, is inviting applications for the position of Project Associate I under a sponsored project in Organic Chemistry. Check eligibility, salary, application process, and important dates here.

Download MPSC Drug Inspector previous year question papers with answer keys. Analyze trends, improve preparation strategy, and boost your exam success.

Check the expected MPSC Drug Inspector Syllabus 2025 PDF, exam pattern, and a comparative analysis of the 2015 and 2022 syllabi for better preparation.

MPSC Drug Inspector Recruitment 2025 – 109 Vacancies in Maharashtra | Salary ₹41,800–1,32,300 | Apply Online before August 21, 2025. Updated Apply Link Here!
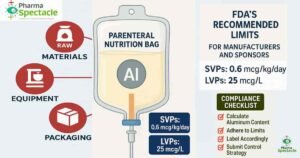
Learn about the FDA’s 2025 draft guidance on aluminum content and labeling recommendations for Small Volume Parenterals (SVPs) and Pharmacy Bulk Packages (PBPs) used in Total Parenteral Nutrition (TPN). Ensure compliance with updated aluminum exposure limits and labeling requirements.

Boost your DMER Pharmacist Exam 2025 success with comprehensive guidance on using previous year question papers effectively, expert study strategies, time management tips.

Apply online for NHM Gujarat Recruitment 2025. 70+ vacancies for Staff Nurse, Pharmacist, Medical Officer & more via Arogyasathi portal. Last date: 12 August.

Apply for 3,500 AIIMS Nursing Officer posts via NORCET-9. Check eligibility, Level-7 salary, age limit, exam pattern, syllabus & apply online.

Explore the latest RRB Paramedical Recruitment 2025 for 434 vacancies. Apply for RRB Pharmacist, Nursing Superintendent, ECG Technician & other posts. Check eligibility, exam pattern & application dates.

The FDA has approved the first generic methimazole-coated tablets to treat hyperthyroidism in cats. Learn how a new veterinary drug works for pet owners and vets.

Discover the complete DMER Pharmacist job profile for 2025. Learn about roles, responsibilities, and salary after selection in the Maharashtra DMER recruitment.

Explore the top private sector Regulatory Affairs job openings in July 2025 at Teva, Syneos, and Amneal. Apply now to accelerate your pharma career!

Sun Pharma and Macleods have recalled specific batches of medicines from the US market due to quality concerns. Here's what pharma professionals need to know.
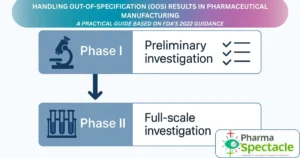
Struggling with out-of-specification (OOS) results in pharmaceutical testing? Learn how to handle OOS investigations step-by-step using FDA’s 2022 guidance.

Preparing for a pharma HVAC interview? Learn how cleanroom systems work, what GMP means in HVAC, and how to answer questions like a pro.

Understand the different types of HVAC systems, including CAV, VAV, Split AC, Central systems, and more. Learn their functions, pros, and uses for industries.

Learn essential HVAC concepts, system types, components, and terms like BTU, SEER, and thermostats. Perfect for beginners and interview preparation.

Apply for 128 vacancies of GSSSB Junior Pharmacist in Gujarat. Check eligibility, exam pattern, salary, syllabus, and get the updated link to apply online before 28 July 2025.
![Why Process Validation Matters in Pharmaceuticals: Purpose, Protocol & Best Practices [2025 Guide] Process validation concept in pharmaceuticals with checklist, pill bottle, and gear icons](https://pharmaspectacle.com/wp-content/uploads/2025/07/Why-Process-Validation-matters-in-Pharmaceutical-Industry-300x158.webp)
In the pharmaceutical industry, patient safety and product quality are based on one key principle: consistency. That’s where Process Validation (PV) steps in—not just as a regulatory checkbox, but as a strategic framework ensuring that every manufactured dose meets rigorous…
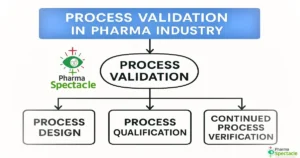
What is process validation in pharma? Learn its definition, lifecycle stages, and GMP guidelines in this comprehensive guide for professionals and students.

Explore category-wise cut-off trends for the DMER Pharmacist exam from 2023. Useful for SC, ST, OBC, and General candidates to estimate target scores.

Explore the latest DMER Pharmacist Syllabus 2025. Discover the latest exam pattern, key topics, and preparation strategy for the Maharashtra DMER Pharmacist recruitment. Get expert tips and essential resources.

DMER invites online applications for 1107 Group C posts, including pharmacists & more. Check DMER registration form 2025 link, eligibility, pay scale & apply.

Master cleaning validation with essential cleaning validation guidelines to ensure GMP compliance, prevent cross-contamination, and uphold product quality.

A complete guide to cleaning validation guidelines, principles, protocols, sampling methods, and practical implementation strategies.

Explore pharmacy as a career in India in 2025! Learn about course options after 12th Science, scope, job opportunities, and expected salary in the pharmacy field.