Process Validation in Pharma: Lifecycle, GMP Guidelines, 2025 Trends & Mistakes to Avoid
What is process validation in pharma? Learn its definition, lifecycle stages, and GMP guidelines in this comprehensive guide for professionals and students.
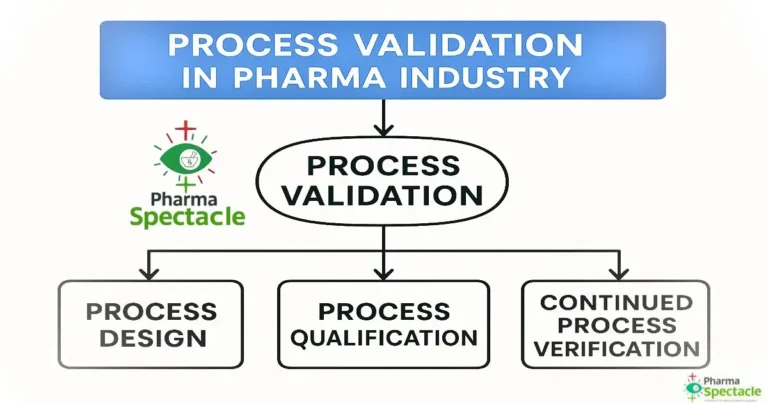
Dive deep into the principles, protocols, and regulatory expectations of process validation in pharmaceuticals. Explore key stages like design qualification, performance qualification, cleaning validation, and ongoing process verification. Stay informed with real-world case studies, FDA, WHO, and ICH guidelines, as well as best practices to ensure quality, compliance, and product safety.

What is process validation in pharma? Learn its definition, lifecycle stages, and GMP guidelines in this comprehensive guide for professionals and students.