![Why Process Validation Matters in Pharmaceuticals: Purpose, Protocol & Best Practices [2025 Guide] Process validation concept in pharmaceuticals with checklist, pill bottle, and gear icons](https://pharmaspectacle.com/wp-content/uploads/2025/07/Why-Process-Validation-matters-in-Pharmaceutical-Industry-1024x538.webp)
In the pharmaceutical industry, patient safety and product quality are based on one key principle: consistency. That’s where Process Validation (PV) steps in—not just as a regulatory checkbox, but as a strategic framework ensuring that every manufactured dose meets rigorous quality standards, batch after batch.
What is Process Validation in Pharma?
Process Validation is a documented evidence-based assessment confirming that a process operates within pre-defined parameters and consistently produces a product that meets its quality attributes. It’s more than proving machinery works—it’s about ensuring that the people, equipment, materials, and methods work harmoniously to deliver a safe, effective product every time
Why Is Process Validation Important?
Process validation ensures that pharmaceutical manufacturing processes are reliable, reproducible, and capable of producing high-quality medicines batch after batch. Here’s why it’s critical:
- Builds confidence for scaling up from pilot batches to full commercial manufacturing.
- Guarantees product consistency
- Improves process understanding
- Reduces batch failures and recalls
- Supports regulatory compliance
- Strengthens patient safety
- Validation also provides a robust QA framework that anchors future audits, investigations, and continuous improvement initiatives.
Whether you’re launching a new facility or changing existing processes, validation proves the manufacturing system is working as intended.
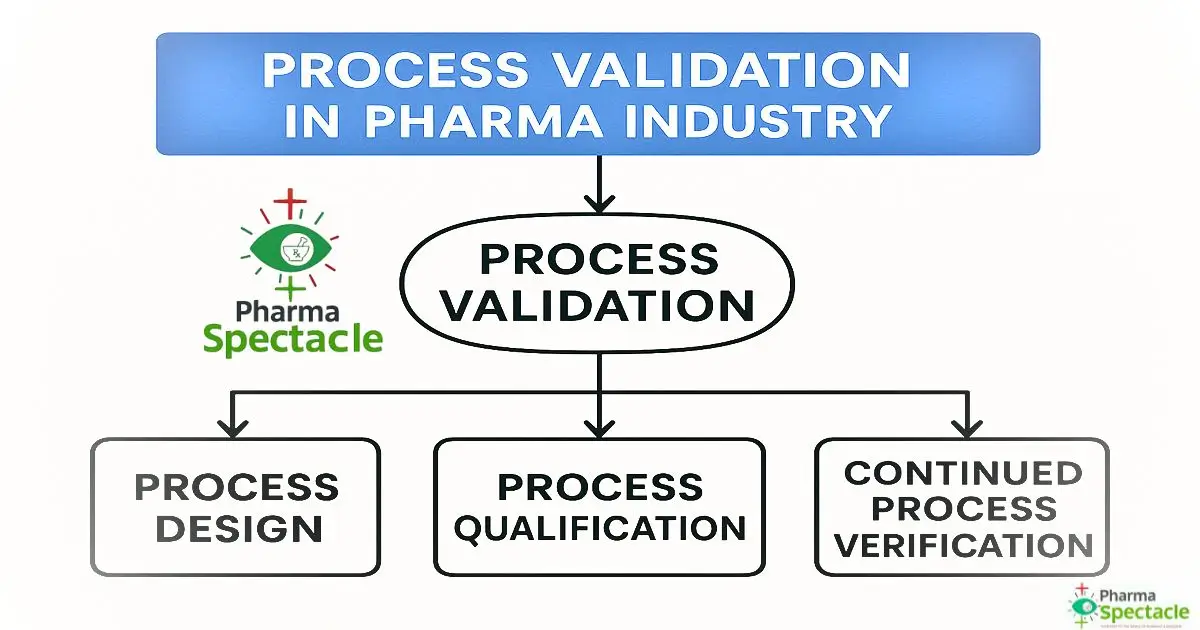
What are the types, stages & documents required for Process Validation?
Click here to read in details types, stages & documents required for Process Validation.
Who Is Responsible for Process Validation?
Process validation requires a cross-functional team from:
- Production
- Process Development
- Engineering
- Quality Assurance
- Quality Control
- Regulatory Affairs
Validation may be handled by internal departments, consultants, or validation committees, but the criteria and regulatory expectations remain consistent.
How to Design an Effective Validation Protocol
An effective validation protocol should answer three key questions:
1. What Is Being Validated?
- Clearly define the product, process, or equipment.
- This ensures focused testing and targeted quality checks.
2. Why Is Validation Needed?
This could be due to new product launches, facility upgrades, regulatory requirements, or changes in raw materials or processes. The reason for validation should be mentioned in the protocol.
3. How Will It Be Validated?
This includes sampling plans, test methods, acceptance criteria, and the number of validation batches.
The validation approach can be either:
- Prospective: Conducted before product is commercially manufactured.
- Concurrent: Performed during actual manufacturing under controlled conditions.
Key validation steps include:
- Defining critical process parameters (CPPs) and critical quality attributes (CQAs).
- Designing test protocols that challenge the system under worst-case scenarios (e.g., minimum & maximum equipment capacity).
- Producing three consecutive validation batches to demonstrate reproducibility.
- Collecting comprehensive data and approving documentation across QA, production, and regulatory teams.
Facility Design and Validation Links
Whether in a new or existing facility, validation must consider:
- HVAC, water systems, material flow, and cleanroom classifications.
- Prior IQ/OQ data and calibration records, if using existing infrastructure.
- Integration of real-time monitoring tools for enhanced process understanding.
How Many Batches Are Required for Validation?
A common industry standard is three consecutive batches manufactured under defined conditions. This number can vary based on:
- Process complexity
- Risk level
- Historical data
- Regulatory expectations
Validation batches should be manufactured by the regular production team, not R&D, to prove the process is robust regardless of operator expertise.
Frequently Asked Questions
1. What is the difference between qualification and validation?
Qualification confirms that equipment and systems operate correctly.
Validation confirms that the process produces the expected output consistently.
2. Can a validated process be changed?
Yes, but any change must undergo a change control procedure, risk assessment, and possibly revalidation depending on the impact.
3. What is a Validation Master Plan (VMP)?
A VMP is a comprehensive document outlining the company’s validation policy, approach, and responsibilities. It includes timelines, systems, and processes requiring validation.
4. Is process validation applicable only to sterile products?
No, validation is required for all pharmaceutical products, whether sterile, non-sterile, solid, or liquid dosage forms.
5. What are Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs)?
CPPs are process variables that impact product quality.
CQAs are physical, chemical, or biological properties that should be within specific limits to ensure product quality.
6. What software or tools are used during validation?
Validation teams may use:
Statistical software like Minitab
Process control software
Electronic Batch Records (EBR) systems
LIMS (Laboratory Information Management Systems)
7. How long does process validation take?
It depends on product complexity, but typically, the initial validation (including protocol preparation, execution, and reporting) may take 3 to 6 months.
8. What is the importance of risk assessment in validation?
Risk assessment (often using tools like FMEA or HACCP) identifies potential failure points in the process and helps focus validation efforts on high-risk areas.
9. What is the role of GMP in process validation?
Good Manufacturing Practices (GMP) are the foundation for process validation. Validation proves the process aligns with GMP requirements to ensure quality, safety, and compliance.
10. What documents are required during process validation?
Key documents include:
Validation Master Plan (VMP)
Protocols and reports for each stage
Batch Manufacturing Records (BMR)
Change control and deviation reports
Final Thoughts: Validation is Not Just a Checklist
Process validation is not a one-time task or a regulatory formality. It’s a living system that must evolve in response to product changes, equipment upgrades, and changing market demands. It’s the discipline that translates development science into manufacturing reality, ensuring that every pill, vial, or capsule is built on repeatable excellence, not chance.
A well-documented and well-executed validation strategy supports regulatory inspections, reduces production risks, and ensures the delivery of safe and effective medicines to patients.
For those building strong QA/QC ecosystems or designing smart validation roadmaps, PV isn’t just a requirement. It’s a commitment to quality that lives far beyond the protocol.
Found this post helpful?
Share it with your friends & colleagues.
Subscribe to PharmaSpectacle to stay ahead in the pharmaceutical world with valuable pharma knowledge and professional growth tips.
Join Our Free Job Alert Channels
- WhatsApp Channel: Click to Join
- Telegram Channel: @PharmaSpectacle
Stay updated with all the latest pharma government job notifications, syllabus, regulatory updates, and many more — all in one place!
Got a question? Drop it in the comments below — we reply to every single one!