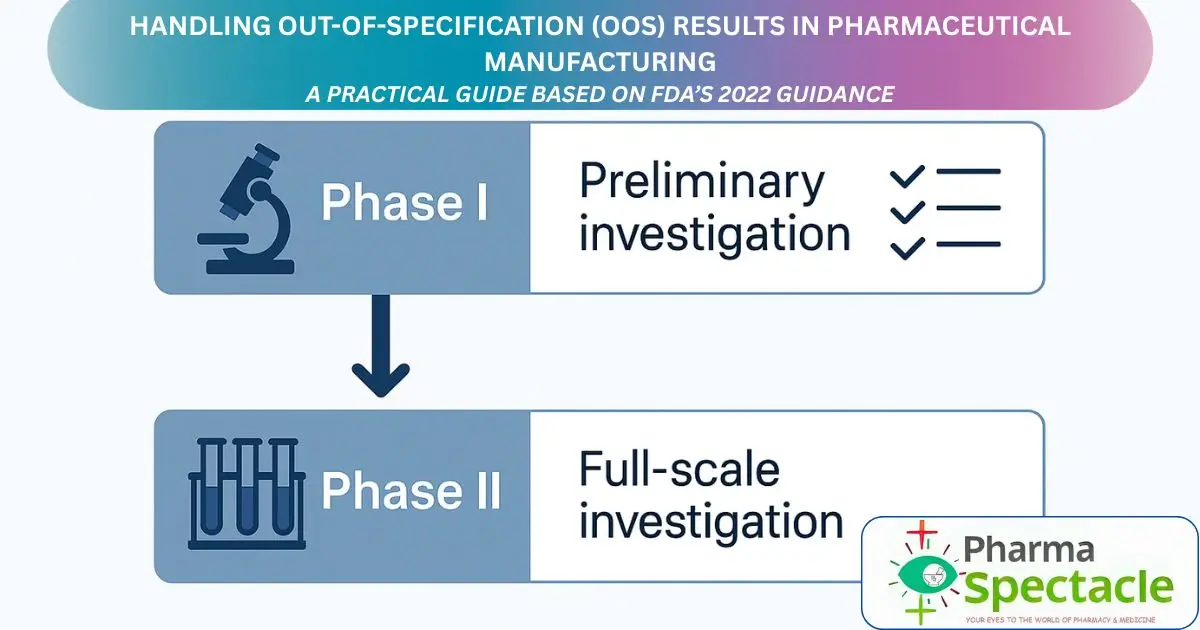
How to Handle Out-of-Specification (OOS) Results in Pharmaceutical Manufacturing – A Practical Guide Based on FDA’s 2022 Guidance
Struggling with out-of-specification (OOS) results in pharmaceutical testing? Learn how to handle OOS investigations step-by-step using FDA’s 2022 guidance.