In pharmaceutical manufacturing, ensuring the quality and safety of drug products is paramount. One of the most critical aspects of quality control is investigating Out-of-Specification (OOS) test results—results that fall outside the predefined specifications set by pharmacopoeias, regulatory filings, or internal company standards. Even a single aberration in lab results—known as an Out-of-Specification (OOS) result—can lead to regulatory setbacks, patient risks, and compromised brand integrity.
The U.S. FDA’s 2022 guidance document, “Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production,” offers a structured, compliant framework for handling such scenarios.
In this article, we break down the FDA’s expectations and translate them into clear, actionable steps for QA, QC, and manufacturing professionals in the pharmaceutical industry.
Also Read: Why Process Validation Matters in Pharmaceuticals: Purpose, Protocol & Best Practices [2025 Guide]
What Are Out-of-Specification (OOS) Results?
An OOS result refers to any analytical testing result that falls outside the established acceptance criteria. These may arise during:
- Raw material testing
- In-process controls
- Finished product analysis
- Stability studies
Importantly, the guidance also applies to in-process tests and contract laboratories and includes both chemical and biological test results.
Understanding OOS Results in Pharmaceutical Testing
As discussed earlier in this article, an Out-of-Specification (OOS) result refers to any analytical test result that falls outside the established acceptance criteria for a pharmaceutical product. These specifications are predefined either by the manufacturer during product development or set by global regulatory authorities such as the FDA, EMA, ICH, or respective national drug regulatory bodies.
For example, if a drug product is required to have 100 mg of active pharmaceutical ingredient (API) per tablet, with an established limit of 100 mg ± 5 mg (95 mg to 105 mg), but during analysis, one tablet is found to contain only 88 mg, this result would be categorized as OOS.
However, it’s important to understand that not every OOS result means there is something inherently wrong with the product. In many cases, the deviation could be due to laboratory errors such as incorrect dilution, instrument malfunction, or sampling mistakes.
That’s why the FDA guidance emphasizes conducting a structured, two-phase investigation to determine whether the OOS result is due to analytical errors or reflects a genuine issue with the batch.
The key takeaway?
A single test result is not the whole story. Only a thorough and well-documented investigation can reveal whether the OOS result is valid, can be invalidated due to lab error, or demands batch rejection due to a confirmed product defect.
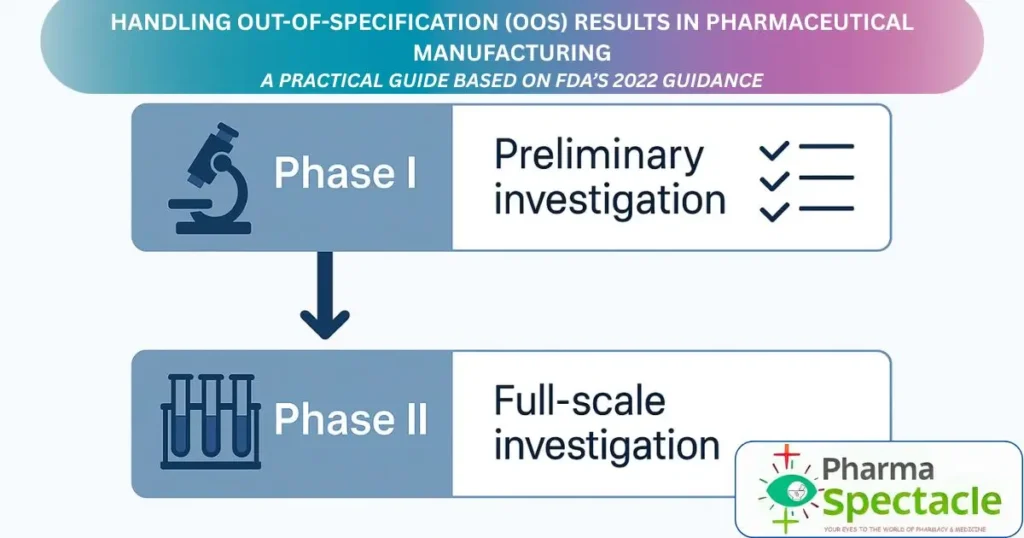
Stages of Investigation
The investigation of OOS results involves two key stages: Phase I – Laboratory Investigation to rule out analytical errors, and Phase II – Full-Scale Investigation to explore potential manufacturing or process-related root causes.
Phase I: Laboratory Investigation – The First Line of Defence.
When an OOS result is observed, the first step is always to investigate within the laboratory. This phase aims to determine whether the result was due to a laboratory error or reflects a real issue with the product.
Responsibilities of the Analyst:
- To ensure the integrity of instruments (calibration, system suitability, performance specifications).
- Check reagents, standards, and sample preparation steps.
- Immediately document any obvious mishaps—e.g., solution spill, sample prep error.
- Immediately report unexpected results to the supervisor and retain test preparations.
- Avoid completing knowingly flawed tests just to see final numbers.
A good analyst doesn’t chase results—they trace the truth.
Responsibilities of the Supervisor:
- Interview the analyst to confirm correct procedure was followed.
- Check & confirm that SOPs and method validations were followed precisely.
- Review raw data meticulously: chromatograms, calculations, reagent quality.
- Confirm instrument performance and reagent quality.
- Ensure traceability for corrective actions in lab logs and QA documents.
If no assignable cause is found, the investigation must proceed to Phase II.
Phase II: Full-Scale OOS Investigation
When lab data appears sound, the focus shifts to manufacturing records, sampling logic, batch variability, and wider product quality implications. This phase involves a broader investigation led by the Quality Unit (QU) and may include manufacturing, engineering, maintenance, and other departments.
Key Steps:
- Review production records for process deviations or trends.
- Assess sampling practices—was the sample representative and handled properly?
- Determine the root cause—whether due to manufacturing, materials, or equipment.
If the OOS result is confirmed and the batch is compromised, reject the batch and extend the investigation to other possibly affected lots.
Production Review
- Examine batch documentation for anomalies.
- Correlate lab results with process development data.
- Ensure trends across batches/products are evaluated—this isn’t a one-batch affair.
Additional Laboratory Testing: Retesting & Resampling
When needed, further testing can be performed, but it must be scientifically justified.
Retesting:
- Retesting uses a portion of the original, homogeneous sample (liquid or solid) that gave the OOS result.
- It is done to check for analytical or handling errors (e.g., suspected dilution errors or instrument issues).
- A second, equally qualified analyst should ideally perform the retest.
- Retesting must follow a predefined SOP specifying the maximum number of allowed retests.
- “Testing into compliance” (retesting until a passing result appears) is strictly prohibited.
- If the retest confirms a lab error, the retest result may replace the original OOS result, but all original data must be retained and documented.
- If no lab error is found, all results—OOS and passing—must be reported and considered during batch disposition.
Retesting isn’t a chance to erase mistakes—it’s a chance to validate integrity.
Resampling:
- Involves taking a new sample from the same batch.
- Only allowed when the original sample is proven non-representative.
- Must follow validated sampling procedures and document justification.
Reporting and Data Interpretation: Transparency Is Key
The FDA emphasizes full data transparency, and one of the most nuanced sections of the guidance revolves around data reporting and statistical treatment.
Appropriate Averaging
- Appropriate only when part of the original method (e.g., multiple injections in HPLC).
- Example: Replicate HPLC injections with acceptance criteria defined in SOP.
Inappropriate Averaging
- Mixing OOS values with in-specification results to generate compliant averages.
- Conceals intra-batch variability, misleads decision-making.
- Inappropriate when used to “hide” OOS results during investigations.
Borderline Values
- Even passing values near the edge of specifications (e.g., 90.1% assay for a 90-110% range) may signal poor formulation intent.
- CGMP requires formulations to aim for ≥100% potency at release to safeguard shelf life.
A “pass” on paper doesn’t guarantee a pass in patient safety.
Outlier Tests
- Can help visualize data dispersion, but cannot be used to invalidate OOS results.
- Especially irrelevant for variability-driven tests like dissolution or content uniformity.
- May be used to identify statistically extreme values.
- Should not be used to invalidate chemical results without root cause analysis.
All individual results—passing or failing—must be recorded and reported to the Quality Unit.
Concluding the Investigation: What the Quality Unit Must Decide
The final verdict rests with the Quality unit, which must consider:
- Confirmed root cause (lab or manufacturing).
- Consistency of retest data.
- Product history and process robustness.
The QA is responsible for interpreting all findings and deciding whether the batch is:
- Rejected – if the OOS result is confirmed.
- Accepted – only if evidence shows the OOS was unrelated to product quality (and documented thoroughly).
- Under Alert – if borderline or low-end values are within spec but suggest formulation or stability concerns.
In cases where the root cause is inconclusive, QA may release the batch only after comprehensive justification, erring on the side of caution. Any batch rejection must trigger a broader failure investigation, potentially across multiple batches or sites.
The QA should never be swayed by convenience—it must be anchored in evidence.
Regulatory Watchouts: Field Alert Reports (FAR)
For products under approved NDAs or ANDAs, if an OOS result from a distributed batch is not invalidated within 3 working days, a Field Alert Report must be submitted to the FDA. This applies to both failing and potentially failing batches (e.g., trending low assay results).
Best Practices for Industry Professionals
- Standardize your OOS SOPs based on the FDA’s framework.
- Train analysts and supervisors to conduct Phase I investigations accurately.
- Ensure robust documentation for every step, especially retesting and root cause analysis.
- Prevent recurrence by tracking trends and implementing CAPA.
- Avoid data manipulation—maintain integrity with full disclosure of all results.
Final Thoughts
The FDA’s 2022 revision of OOS investigation guidance underscores one truth: data integrity and scientific investigation are the backbone of pharmaceutical quality. Whether you’re in QC, QA, regulatory affairs, or manufacturing, understanding how to handle OOS results can protect patient safety, company reputation, and regulatory compliance.
Implement these practices proactively, and your organization will be well-prepared for inspections, audits, and above all, quality excellence.
Found this post helpful?
Share it with your friends & colleagues.
Follow us on WhatsApp & Telegram for job updates, interview tips, and study material:
Subscribe to PharmaSpectacle to stay ahead in the pharmaceutical world with valuable pharma knowledge and professional growth tips.
Published by PharmaSpectacle – Your trusted lens into pharmaceutical careers and compliance.




