In July 2025, the U.S. Food and Drug Administration (FDA) issued a revised draft guidance titled “Small Volume Parenteral Drug Products and Pharmacy Bulk Packages for Parenteral Nutrition: Aluminum Content and Labeling Recommendations.” This replaces the 2022 version and is open for public comment until September 2, 2025.
This draft, published in July 2025 under Docket No. FDA-2022-D-2301 addresses the key safety concern: aluminum toxicity in patients receiving parenteral nutrition (PN), especially neonates and individuals with renal impairment. Since the body’s ability to eliminate aluminum is limited in vulnerable populations, even small amounts can accumulate and cause serious health issues such as bone demineralization and neurological problems.
Click here to check: FDA Approves First Generic Coated Tablets for Cats with Hyperthyroidism
Key Highlights of the 2025 Draft Guidance
- Total Aluminum Exposure (TAE) from PN should not exceed 5 mcg/kg/day.
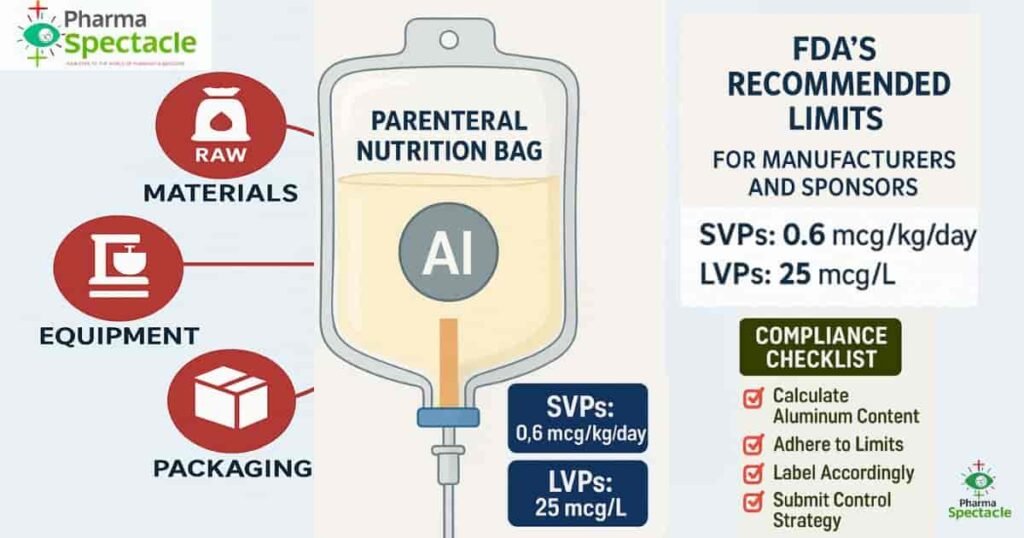
- Large Volume Parenterals (LVPs) must contain no more than 25 mcg/L of aluminum.
- Small Volume Parenterals (SVPs) and PBPs are required to calculate and label aluminum content based on Individual Aluminum Exposure (IAE) and Aluminum Concentration Limits (ACL).
- Labeling requirements include specific warnings and aluminum content disclosures on both prescribing information and packaging.
- Manufacturers are required to use validated assay methods and submit aluminum control strategies in their NDAs or ANDAs.
Aluminum exposure in PN primarily comes from:
- Raw material contamination,
- Manufacturing equipment leachables,
- Packaging materials like Aluminum Bags, glass vials and stoppers.
Prolonged exposure can lead to severe health issues like:
- Osteomalacia and bone demineralization,
- Neurotoxicity (dialysis encephalopathy),
- Microcytic anemia,
- Cholestasis in neonates.
The FDA’s target is to ensure that total aluminum exposure (TAE) does not exceed 4-5 micrograms per kilogram per day (mcg/kg/day) in PN therapy, beyond which toxicity may occur.
1. Aluminum Concentration Limits (ACL) for SVPs
While Large Volume Parenterals (LVPs) are capped at 25 mcg/L aluminum content by regulation (21 CFR 201.323), SVPs lacked a clear statutory limit. This guidance recommends that individual SVPs should not contribute more than 0.6 mcg/kg/day to the Total Aluminum Exposure (TAE), allowing up to 5 SVPs to be safely combined in PN admixtures.
“Join our WhatsApp and Telegram Groups to get the latest pharmacy job updates, industry news, and expert insights—directly from PharmaSpectacle!”
2. Calculation of ACL for SVPs
Manufacturers must calculate the Aluminum concentration limit (ACL) in SVPs using standardized formulas, considering:
- Drug concentration (mg/mL).
- Maximum daily dosage (mg/kg/day).
- Selected individual aluminum exposure (IAE) limit.
Example: For Zinc Chloride Injection, ACL is calculated to ensure TAE remains within the safety threshold.
3. Manufacturing & Control Strategies
The guidance emphasizes:
- Validated testing methods for aluminum content.
- Manufacturing controls to minimize leachable.
- Packaging considerations (especially glass containers).
- Quality Target Product Profile (QTPP) incorporating aluminum limits.
4. Labeling Requirements
Updated recommendations include:
- Aluminum content declaration on container labels and cartons.
- Specific warnings in Prescribing Information regarding aluminum toxicity risks.
- Pediatric-specific precautions.
- Cross-referencing in the Description Section to inform healthcare professionals.
Industry Impact and Compliance Timeline
- This guidance will apply to all new NDAs, ANDAs, and supplements for SVPs and PBPs used in PN.
- Sponsors of existing marketed products are advised to perform risk assessments and revise specifications within two years post-finalization of the guidance.
- Failure to control aluminum levels could lead to regulatory actions, including product recalls or market withdrawals.
Authoritative Sources for Reference:
- FDA’s New Draft Guidance for Parenteral Nutrition Packaging
- FDA Guidance Documents Portal
- 21 CFR 201.323 – FDA Regulations on Aluminum in LVPs
- ICH Q3D(R2) Guidelines on Elemental Impurities
- USP General Chapters <7> & <659>
Why Compliance Is Critical
Meeting these guidance recommendations isn’t just about regulatory compliance—it’s about protecting the most vulnerable patients from preventable harm. Updating manufacturing, testing, and labeling practices will ensure that hospital and clinical stakeholders are assured that products are held to the highest standards.
Found this post helpful?
Share it with your friends & colleagues.
Follow us on WhatsApp & Telegram for job updates, interview tips, and study material:
- Join WhatsApp Channel
- Join Telegram Channel
Subscribe to PharmaSpectacle to stay ahead in the pharmaceutical world with valuable pharma knowledge and professional growth tips.
Published by PharmaSpectacle – Your trusted lens into pharmaceutical & Medical careers and compliance.